What is the Electron Configuration of Oxygen Archives Dynamic Periodic Table of Elements and
How to Write Ground State Electron Configuration in Chemistry
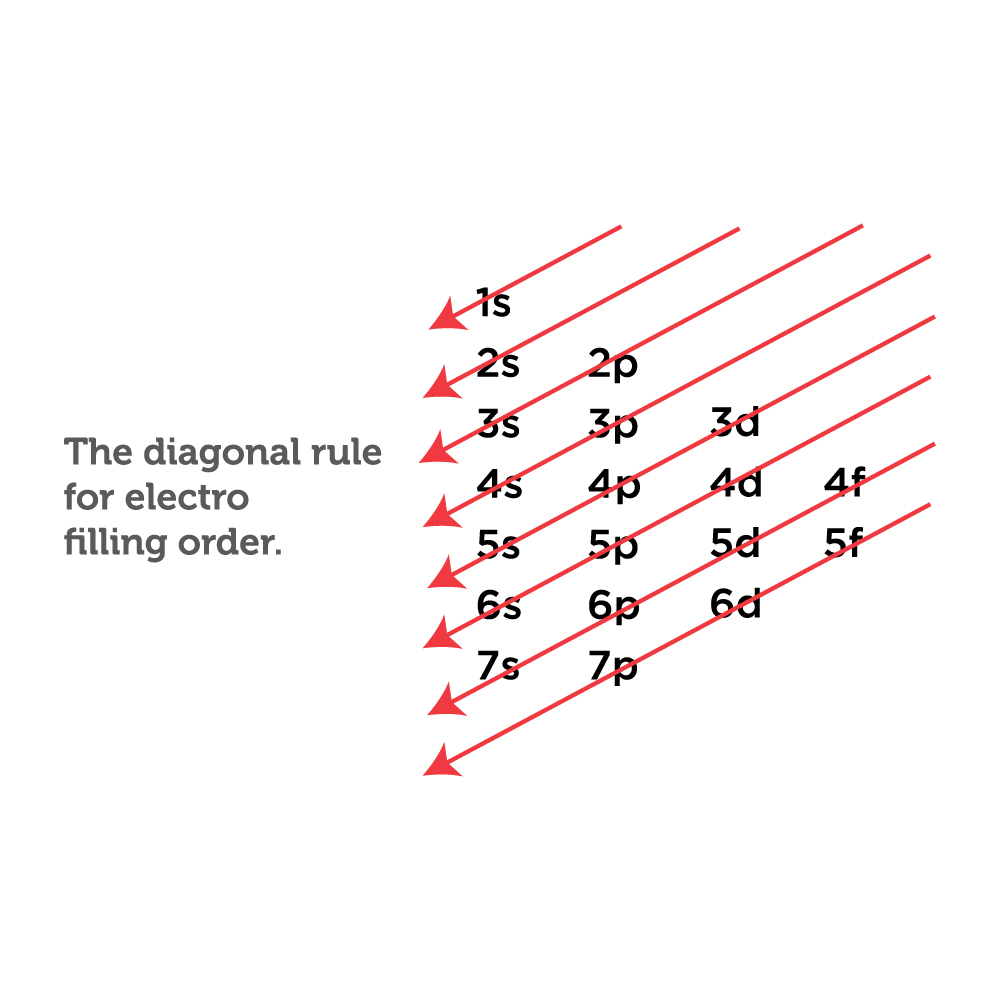
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 3.1.2 3.1. 2 ): The number of the principal quantum shell, n, The letter that designates the orbital type (the subshell, l ), and

Symbol and electron diagram for Oxygen Royalty Free Vector
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.

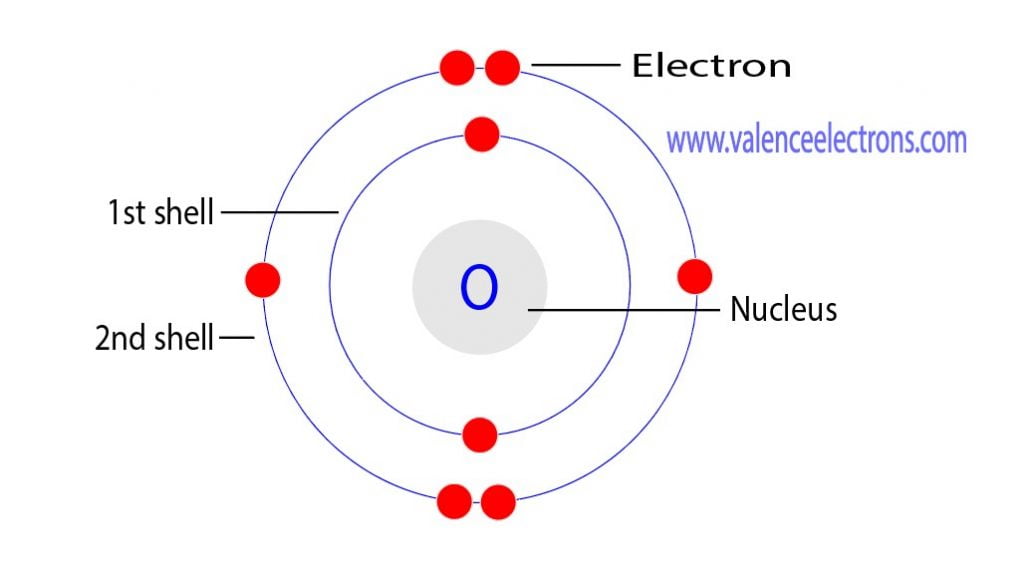
FileElectron shell 008 Oxygen.svg Wikimedia Commons Atom diagram, Electron configuration
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
Oxygen(O) electron configuration and orbital diagram (2022)
Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons occupy. Ground state means that the atom has the lowest energy allowed. The electron configuration is responsible for many physical and chemical properties of an element.
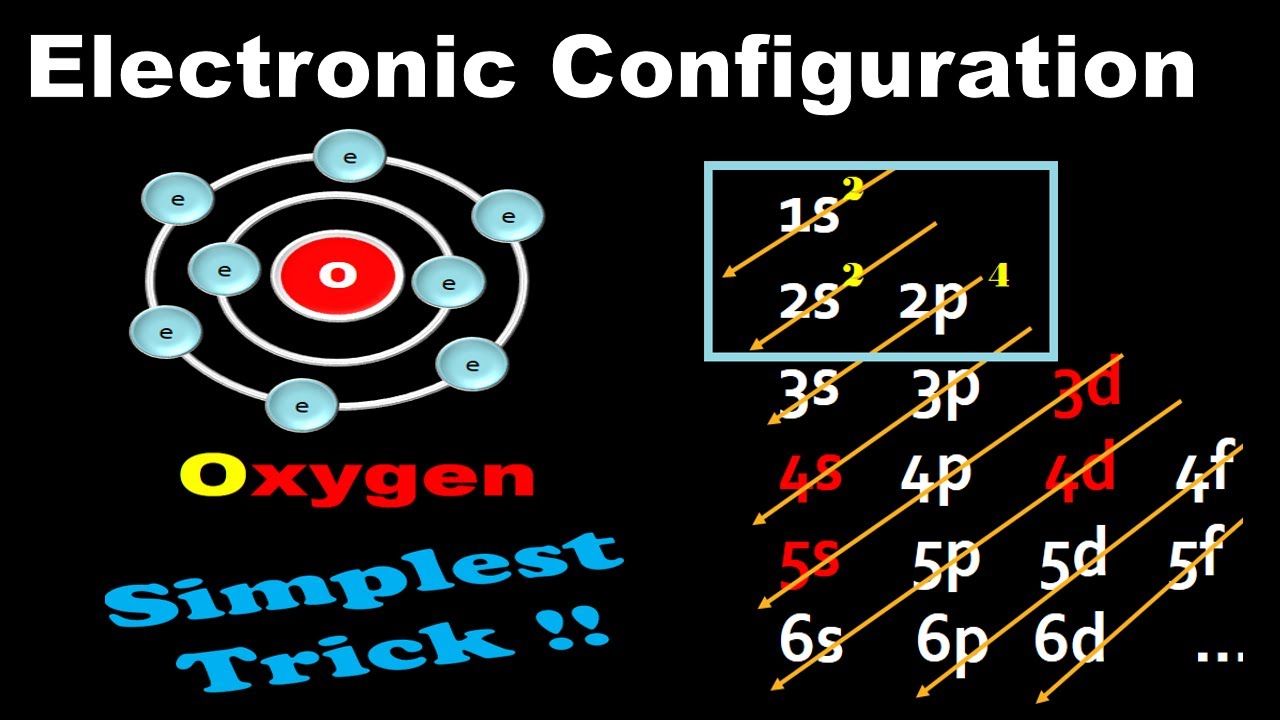
Electronic Configuration For Oxygen spdf Trick Chemistry Atomic Number 8 YouTube
Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Oxygen: The electronic configuration of Oxygen is 1 s 2 2 s 2 2 p 4. Oxygen requires two electrons to attain noble gas configuration. Suggest Corrections 24
Bohr Model Oxygen Chemical Element Atomic Number, PNG, 1000x1000px, Bohr Model, Area, Atom
The electron configuration of an oxygen atom [He] 2s 2 2p 4 suggests that neutral oxygen atoms can achieve an octet of valence electrons by sharing two pairs of electrons to form an O=O double bond, as shown in the figure below. According to this Lewis structure, all of the electrons in the O 2 molecule are paired.
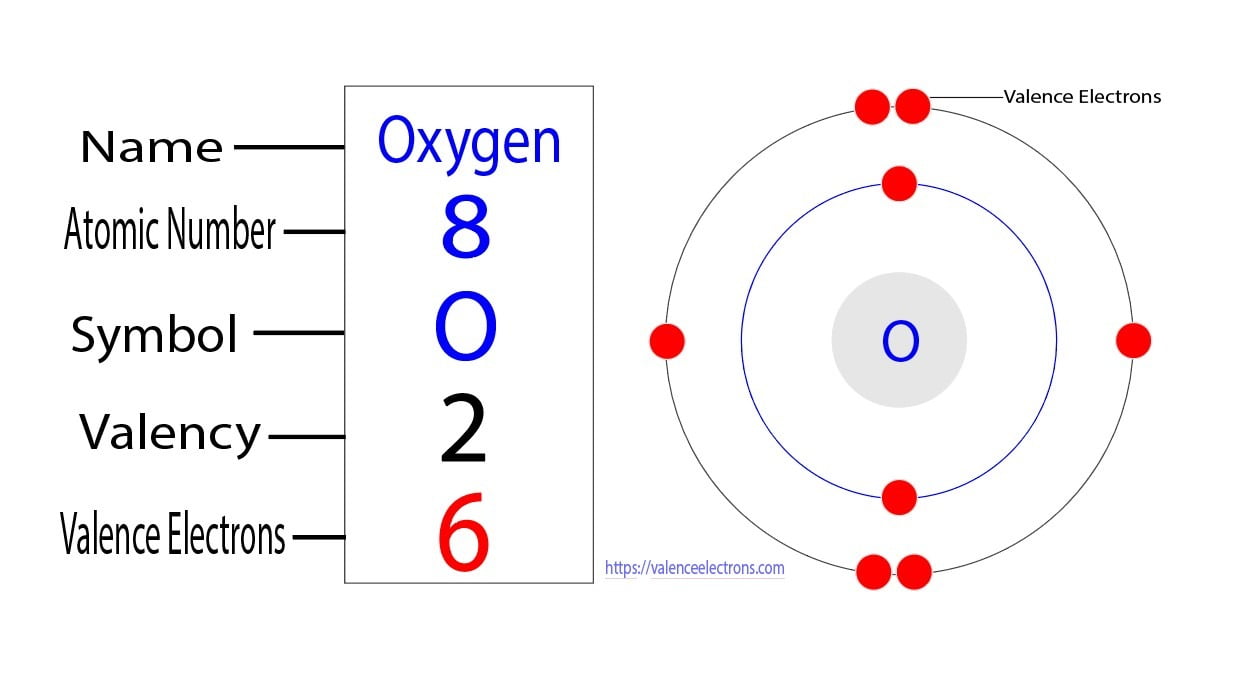
How to Find the Valence Electrons for Oxygen (O)?
Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen's electron configuration would be O 1s 2 2s 2 2p 4. Special Cases. Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place.

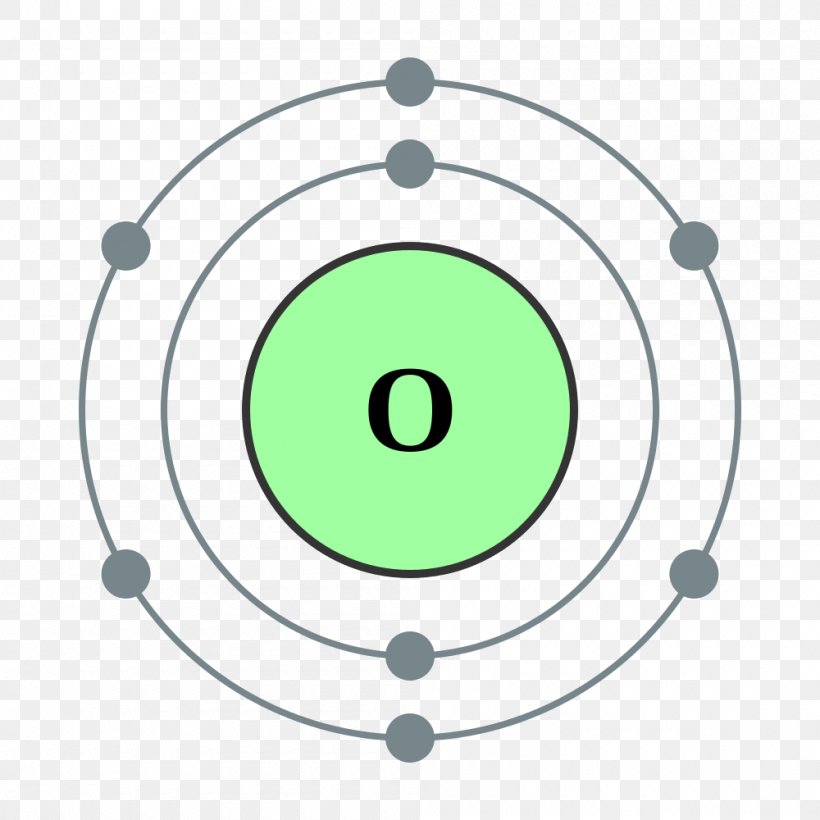
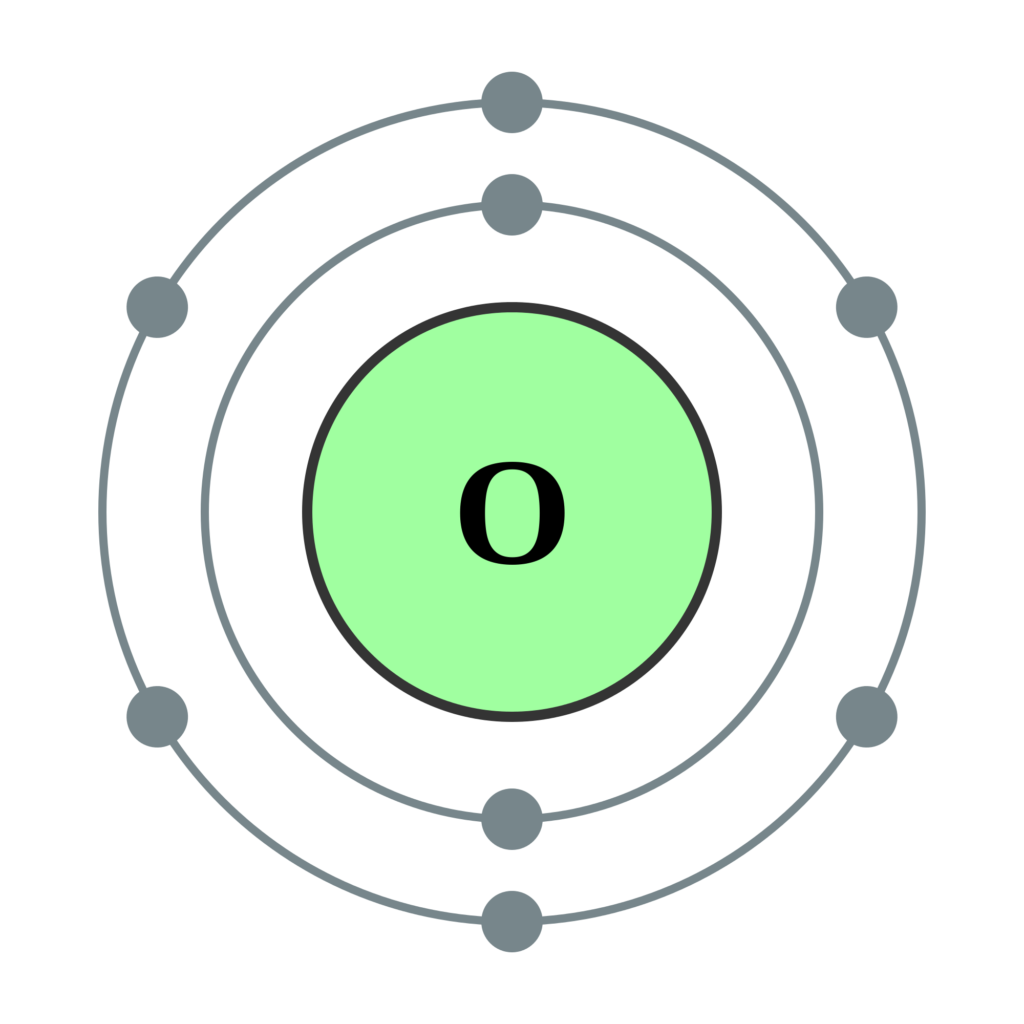
Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
Oxygen - Element information, properties and uses | Periodic Table Pressure and temperature data - advanced Young's modulus (GPa) Shear modulus (GPa) Bulk modulus (GPa) , the magazine of the Royal Society of Chemistry. O, just about the most perfect solvent you can imagine for biochemistry.

The electron configuration of oxygen is 1s2,2s2 2p4. Science chemistry, Electron configuration
Let's find the electron configuration of Oxygen! A single oxygen atom has 8 protons and 8 electrons, but how do we know where Oxygen puts its electrons, in w.
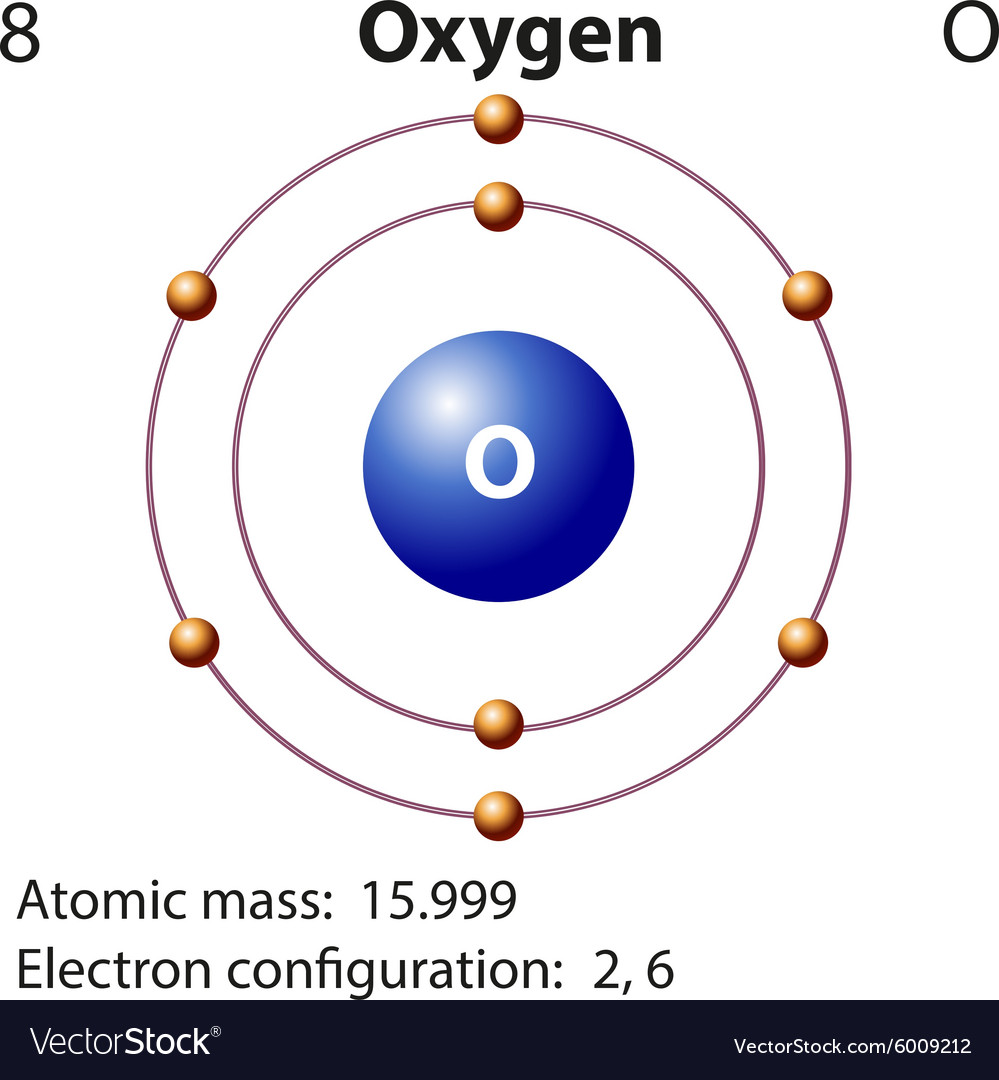
Diagram representation element oxygen Royalty Free Vector
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write Electron Configurations. Step-by-step tutorial for writing the Electron Configurations.

Oxygen Atom Science Notes and Projects
The electronic configuration of oxygen is- 1s22s22p4 Note:- For writing the electronic configuration of elements, the Aufbau Principle is used. In Aufbau Principle, the electrons are filled according to the increasing energy level of orbitals.
What is the Electron Configuration of Oxygen Archives Dynamic Periodic Table of Elements and
A step-by-step description of how to write the electron configuration for Oxygen (O). In order to write the O electron configuration we first need to know t.

√ Electron Configuration Of Oxygen Ion Lir Stardust
In this video we will write the electron configuration for O 2-, the Oxide ion. We'll also look at why Oxygen forms a 2- ion and how the electron configurati.

Electron Configuration for Oxygen (O, O2 ion)
Ground State Electron Configuration of Oxygen. The way electrons are arranged in oxygen is shown by the numbers 1s^2, 2s^2, 2p^4. This tells us how many electrons are in each part. Let's break it down and explain it more simply. Oxygen has eight electrons. The first energy level can hold two electrons, and oxygen has two at this level.
Oxygen Electron Configuration (O) with Orbital Diagram
If we look at the element after nitrogen in the same period, oxygen (Z = 8) its electron configuration is: 1s 2 2s 2 2p 4 (for an atom). Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. Occupation of Orbitals.
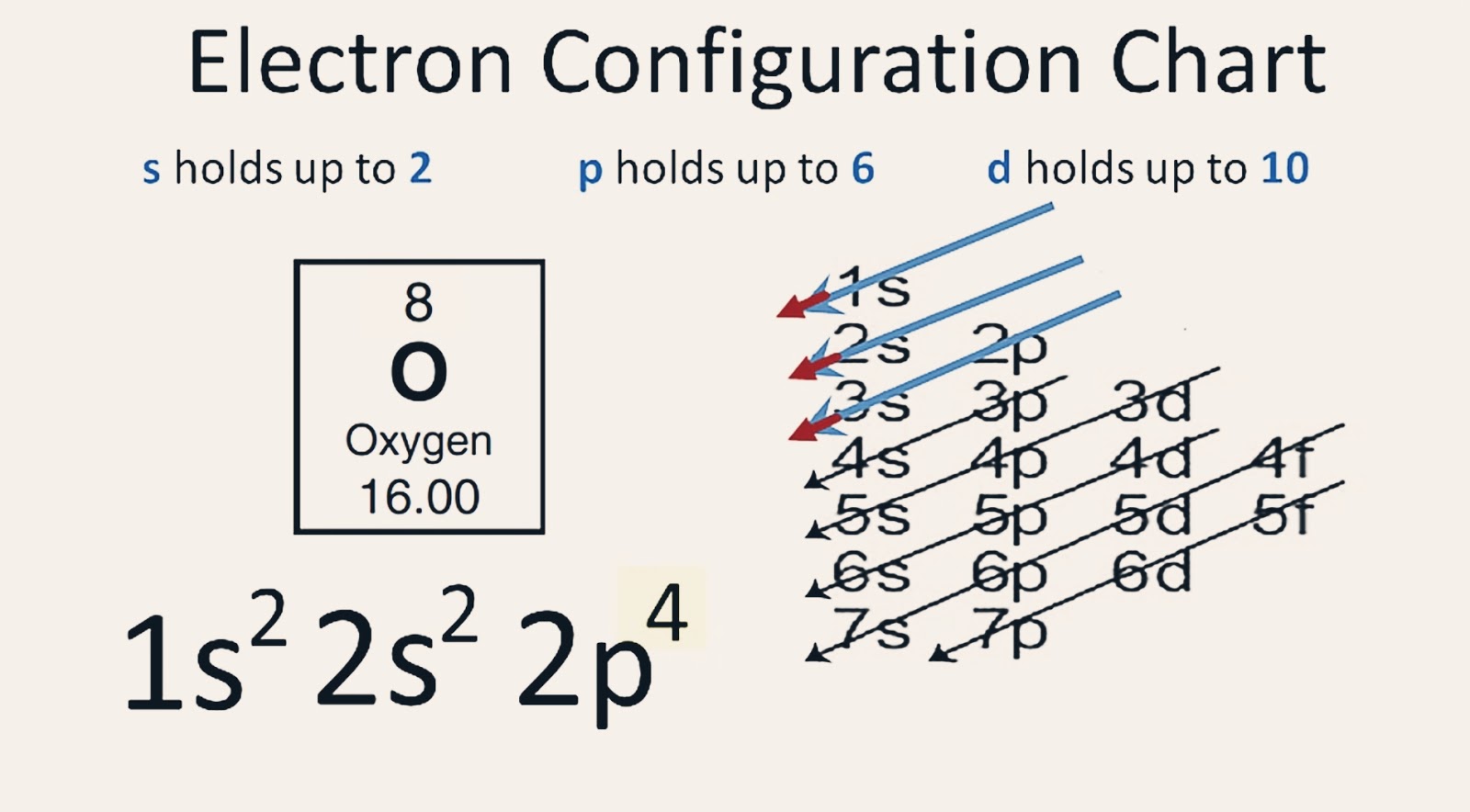
What Is the Oxygen Electron Configuration(O)?
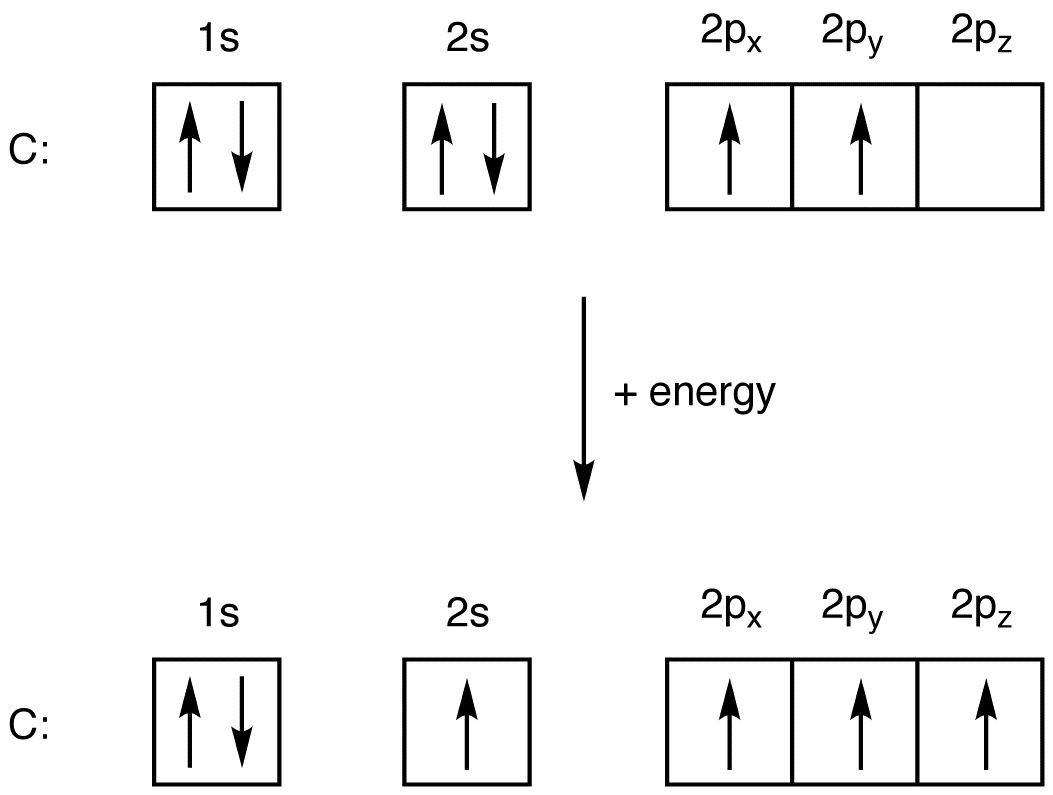
Example 1.6.3 1.6. 3: Carbon and Oxygen. Consider the electron configuration for carbon atoms: 1s 2 2s 2 2p 2: The two 2s electrons will occupy the same orbital, whereas the two 2p electrons will be in different orbital (and aligned the same direction) in accordance with Hund's rule. Consider also the electron configuration of oxygen.